Sunitinib Malate
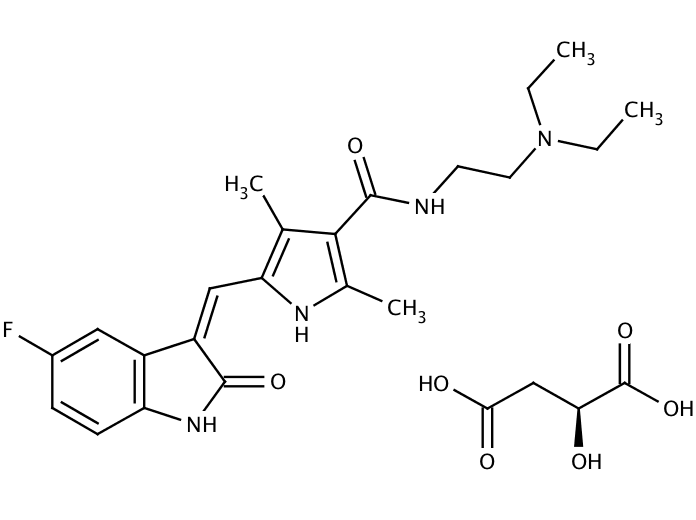
Sunitinib is an oral, small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) on January 26, 2006. Sunitinib was the first cancer drug simultaneously approved for two different indications
Product Details
| Category |
API's & Drug Standards Cancer Research and Antivirals |
|---|---|
| Price | POA |
| CAS Number | 341031-54-7 |
| Formula | C22H27FN4O2 · C4H6O5 |
| Molecular Weight | 532.56 |
| Purity | 98.0% |
| Appearance | powder form |
| Availability | In Stock |
| Storage Conditions | +4°C |
Product Enquiry
Send us your enquiry for Sunitinib Malate. We offer custom pack sizes at special prices. We aim to respond to your enquiry within 24 hours.
We value your input so if you have suggestions regarding new applications for Sunitinib Malate email us and we will include your contribution on the website.



